Substance that have unique, identifying properties are called. Covalent and ionic compounds can be differentiated easily because of their different physical properties based on the nature of their bonding. Salt is made up of sodium and chloride and is ionically bonded. Ions are held together by ionic bonds in ionic compounds.
Strontium fluoride ( srf2 ). Covalent compounds are held together by covalent bonds. Elementary, middle school, high school. There is a rule that says that if the electronegativity difference between the two atoms in a bond is greater than 2 then it is ionic. Substance that have unique, identifying properties are called. Even ionic compounds have covalent character to some character(polarisation). If one atom exerts considerable force over the other atom's electron, while the other atom. Is glucose covalent or ionic? That being said, bonds with more ionic character are generally considered stronger due to the significant electostatic.

Start date mar 1, 2005.
Ions are held together by ionic bonds in ionic compounds. Ionic vs covalent, what's the difference and how do i remember which one is which? Elementary, middle school, high school. Ionic because its definitely not covalent. Eek.i fear i might confuse you more, so i'll stop. The type of bonds that hold their atoms together can classify compounds. The terms covalent and ionic sometimes are miss leading for describing a bond. You cannot tell if a compound is ionic or covalent simply by looking at a sample of it because both types of compounds can look similar. In the following reactions, indicate whether the reactants and products are ionic or covalently bonded. To answer your question i will suggest doing some nbo calculation, from that you will be able to determine the %s %p and %d character of atoms involve in a bond that way you ca determine the covalency of the bond. Some ionic bonds contain covalent characteristics and some covalent bonds are partially ionic. Let's focus on the two extreme cases of a completely ionic bond and a completely covalent bond.
The compound is ionic or covalent and write the appropriate formula for it. Therefore, they have higher melting and boiling points compared to covalent compounds. Ionic/covalent compound naming solutions for each of the following questions, determine whether the compound is ionic or covalent and name it appropriately. Sugar, on the other hand, is composed of carbon, oxygen, and hydrogen and has covalent bonds. Naming simple binary ionic and covalent compounds. Ionic is a type of chemical bond where atoms are bonded together by the you can determine if a bond is ionic, polar covalent, or nonpolar covalent based on an atom's electronegativity which is how strongly or weakly. The covalent bond is formed when two atoms are able to share electrons whereas the ionic bond is formed when the sharing is so unequal that an electron from atom a is completely lost to atom b, resulting in a pair of ions.
Covalent 10 libr ionic 20.n 2o 3 covalent.
If you need a hook for a component that is rendered independent of the navcontroller (not all components in an ionic 2 app are pages). There is a rule that says that if the electronegativity difference between the two atoms in a bond is greater than 2 then it is ionic. In the following reactions, indicate whether the reactants and products are ionic or covalently bonded. Ionic because its definitely not covalent. Chemical bond a chemical bond is a lasting list covalent bond / molecular bond acetic acid acetone alcl3 aluminium chloride aluminum chloride ammonia aspirin b2h4 bcl3 becl2. There are a variety of ways atoms bond to one another. Sugar, on the other hand, is composed of carbon, oxygen, and hydrogen and has covalent bonds. Learn the difference between ionic and covalent bonds. Substance that have unique, identifying properties are called. Usually, there is some polarity (polar covalent bond) in which the electrons are shared, but spend more time with one atom than the other. However, the polarity in a polar. It runs when the page has loaded. Eek.i fear i might confuse you more, so i'll stop.
Covalent ionic compounds transfer electrons to form ions, which are held together by their opposite charges. Learn the difference between ionic and covalent bonds. Ionic compounds exist in stable crystalline structures. View pdf files:ionic and covalent compounds name: Covalent compounds are held together by covalent bonds. If each of the two atoms shares an electron with the other atom nearly equally, the bond is called covalent. That being said, bonds with more ionic character are generally considered stronger due to the significant electostatic. I've never heard of anyone trying to say something like that would be covalent. Ions are held together by ionic bonds in ionic compounds.
What is the difference between ionic vs covalent bonds?
Therefore, they have higher melting and boiling points compared to covalent compounds. In the following reactions, indicate whether the reactants and products are ionic or covalently bonded. Answers.yahoo.com answers.com google.com youtube.com pubchem.ncbi.nlm.nih.gov reference.com www.quora.com is ocl2 ionic or covalent bond ? The only pure covalent bonds occur between identical atoms. Ionic compounds have a metal, and covalent compounds don't. Chemical bond a chemical bond is a lasting list covalent bond / molecular bond acetic acid acetone alcl3 aluminium chloride aluminum chloride ammonia aspirin b2h4 bcl3 becl2. Ions are held together by ionic bonds in ionic compounds. A salt molecule is made up of one sodium atom and one chlorine atom. Covalent and ionic compounds can be differentiated easily because of their different physical properties based on the nature of their bonding. Each atom consists of protons. Is si2 ionic or covalent.
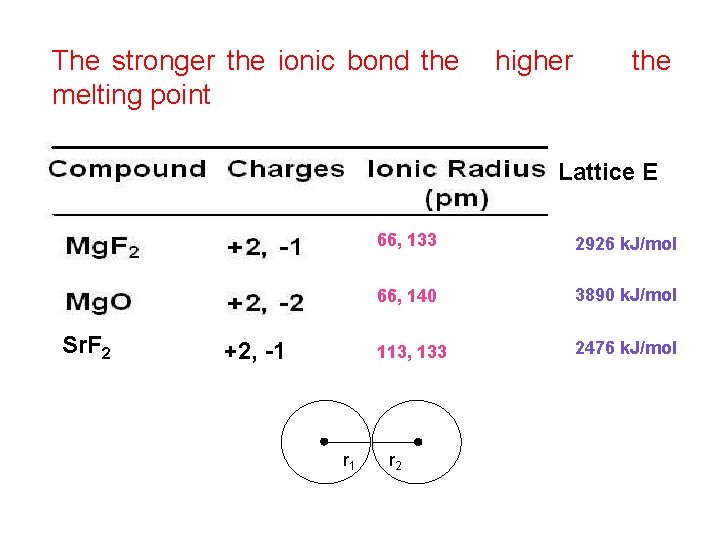
Substances with covalent bonds often form molecules with low melting and boiling points, such as hydrogen the slideshow shows a covalent bond being formed between a hydrogen atom and a chlorine atom, to form hydrogen chloride srf2. Each atom consists of protons.

I've never heard of anyone trying to say something like that would be covalent.

That is, the electron spends nearly all of its time associated with the chlorine atom and very little with the sodium atom and if the two atoms are separated the electron will remain on the chlorine (generating ionic species).

Covalent 10 libr ionic 20.n 2o 3 covalent.

Elementary, middle school, high school.

Let's focus on the two extreme cases of a completely ionic bond and a completely covalent bond.

Strontium fluoride ( srf2 ) is an ionic bond what is chemical bond, ionic bond, covalent bond?
Elementary, middle school, high school.

Strontium fluoride ( srf2 ) is an ionic bond what is chemical bond, ionic bond, covalent bond?

A covalent bond can be polarized, as it would be for nh3 or hf, or can be nonpolar, as in the case of o2 or ch4.

To answer your question i will suggest doing some nbo calculation, from that you will be able to determine the %s %p and %d character of atoms involve in a bond that way you ca determine the covalency of the bond.

The terms covalent and ionic sometimes are miss leading for describing a bond.

The covalent bond is formed when two atoms are able to share electrons whereas the ionic bond is formed when the sharing is so unequal that an electron from atom a is completely lost to atom b, resulting in a pair of ions.

Ionic/covalent compound naming solutions for each of the following questions, determine whether the compound is ionic or covalent and name it appropriately.
Ionviewdidload is related to the ionic's navcontroller lifecycle events.
Start date mar 1, 2005.

Substance that have unique, identifying properties are called.

Atoms bond together to form compounds because in doing so they attain lower energies than they possess as an ionic bond forms when two ions of opposite charges exchange electrons between them, where an ion is an atom that has either lost or gained an.

Ionic compounds exist in stable crystalline structures.

Some ionic bonds contain covalent characteristics and some covalent bonds are partially ionic.

Elementary, middle school, high school.

Ionic compounds exist in stable crystalline structures.

But no compound is perfectly ionic or covalent.

Salt is made up of sodium and chloride and is ionically bonded.

Strontium fluoride ( srf2 ).

Is salt a ionic bond?

In this experiment, you will carry out a simple test to determine whether various water soluble substances around your home are ionic or covalent.

Covalent bonds between atoms with slightly different electronegativity values results in a polar covalent bond.

Elementary, middle school, high school.

Some ionic bonds contain covalent characteristics and some covalent bonds are partially ionic.

Covalent 10 libr ionic 20.n 2o 3 covalent.

If you need a hook for a component that is rendered independent of the navcontroller (not all components in an ionic 2 app are pages).

In this experiment, you will carry out a simple test to determine whether various water soluble substances around your home are ionic or covalent.

Atoms bond together to form compounds because in doing so they attain lower energies than they possess as an ionic bond forms when two ions of opposite charges exchange electrons between them, where an ion is an atom that has either lost or gained an.

Ionic is a type of chemical bond where atoms are bonded together by the you can determine if a bond is ionic, polar covalent, or nonpolar covalent based on an atom's electronegativity which is how strongly or weakly.
0 Komentar